Vaccine distrust is one legacy of unethical Big Pharma practices in Nigeria
- Written by Mahdi Garba and Modupe Abidakun
- Edited by Mercy Abang
- Illustration by Antoine Bouraly
The story was published by our partner Unbias the News.
In Kano State, Nigeria, vaccination hesitancy is not only driven by conspiracy theories or mistrust in science. As health advocates struggle to fight disinformation about the COVID-19 vaccines, it is important to remember that in some countries distrust stems not only from ignorance, but also from lived experience.
12 July 2021
“It’s strange that I still remember everything, even the color of the nurse’s uniform. There was a white nurse who was in a brown skirt and green blouse, who directed the Nigerian nurse to give him three injections at a time and he did exactly that while my son was on my shoulders,” says Maryam*, speaking in the local Hausa dialect.
The mother of now 28-year-old Zakari, Hajiya Maryam, recounts how the Pfizer drug test in Kano left her son with a speech and hearing impairment.
“My son was six years old then. He was ill and when I saw the symptoms: fever and headache, they looked like meningitis,” she tells Unbias the News. “So, I took him to a clinic– Asibitin Zana–where on arrival I saw a host of Nigerian nurses alongside other white people”.
“Immediately after receiving the drugs, he became unconscious for hours. On waking up, I noticed that he couldn’t hear anything again – I knew that it was Pfizer who gave him the drugs”. Maryam insists no one told her that Pfizer was testing out a new drug.
The story of a 1996 Pfizer drug trial and its outcomes and aftermath is linked to the current COVID-19 vaccination boycott in communities like Kano State – vaccine hesitancy does not only apply to situations where vaccine uptake is low because of poor availability but also because of distrust and safety concerns – based on experience.
Drug Trials During an Outbreak
In June 1996, a severe meningitis outbreak rocked Nigeria, an infection that caused inflammation of the brain and spinal cord linings and spread through sneezing.
As of March 4, 1996, the infection had spread to 12 states in Nigeria in the space of six months, leading to over 100,000 cases and a fatality rate of 10.7% – meaning it caused death in one in every ten patients with the infection. It was the most severe epidemic of the illness ever recorded in Nigeria.
The outbreak, which lasted over three months, saw the combined efforts of a National Task Force set up by Nigeria’s Federal Ministry of Health, the WHO, UNICEF, UNDP, Médecins Sans Frontières, the International Red Cross and several other non-governmental organizations to bring the epidemic under control, but not without scars left behind for some families in Kano State.
In addition to the international task force, the US-based pharmaceutical company Pfizer was in Kano at the time with an antibiotic drug called Trovan, expected to potentially treat meningitis, but not yet approved for that use or for treatment of children by the US Food and Drug Administration (FDA). The company administered a drug trial of Trovan and a second drug, Ceftriaxone, then a standard treatment for meningitis, to some 200 children.
A Question of Consent
Pfizer has maintained that they obtained prior verbal consent from all parents for the experiment, but parents like Maryam and trial participants like 29-year-old Bala Bello tell a different story.
Bello, a graduate of Business Administration from Bayero University Kano, was four- years-old during the meningococcal meningitis outbreak. His hometown was the epicenter of the outbreak, which left scores dead in Kano State.
Bello recounted what his mother told him happened. “I was ill and taken to the Infectious Diseases Hospital (IDH), popularly known as ‘Asibitin Zana’ located along France Road in Kano”, he explained. “I was given some drugs, which no one explained to her (my mum) what the said drugs were meant for.”
Bello’s mum had assumed the drugs were for the treatment of meningitis, but shortly after the drugs had been administered to him he developed an unexpected side effect.
“We didn’t even leave the hospital before a reaction manifested. Soon after, I developed paralysis in my legs,” Bello says, while struggling to maintain a stable sitting position during the interview.
It was “soon after I was paralysed, that my mother got to know that it was Pfizer who had given her the drugs from their experiment,” Bello recalls.
Of the participants in the trial, 11 children died and dozens of others were left with debilitating injuries: blindness, paralysis, deafness, and neurological deficits, which the company maintains are the result of meningitis, not the drugs they administered. (Pfizer did not respond to multiple requests for comment for this story.)
In 1998, the license for Trovan for use by adults was withdrawn from the European Medical Agency, because of concerns over serious medical problems and multiple deaths. It was withdrawn from the US market in 1999 for the same reasons, though at the time Pfizer said trials had revealed no side effects. It appears results from the trial in Kano State were never published.
What followed in the years after the drug trial would be a lengthy legal battle between Pfizer, the Nigerian government, and the families of the children involved.
Statement from the European Medicines Agency, 1999
“The scientific Committee for Proprietary Medicinal Products (CPMP) of the European Medicines Evaluation Agency (EMEA), during its extraordinary meeting on 10 June 1999, adopted an Opinion recommending the suspension of the marketing authorisation of TROVAN/TROVAN IV and TURVEL/TURVEL IV. This was due to increasing concerns over 152 documented reports of serious hepatic events, including 9 cases where patients died or required a liver transplant.”
From Drug Trial to Legal Trial
In June 2007, the Nigerian federal government and the Kano State government filed criminal and civil suits against Pfizer and eight other defendants, asking for $7 billion in damages. The suit charged that the company had tested an unapproved and experimental drug on children with neither informed consent from parents, nor approval from the Nigerian government. Pfizer countered that such approval wasn’t necessary.
“Pfizer contends that there was no regulation or law in Nigeria requiring ethical committee approval before conducting a clinical trial or investigative study. Therefore, there was no need to obtain what the law did not require.” – From Pfizer’s Defense in Kano State Civil Case
In 2001, an investigation by the Washington Post uncovered that a document Pfizer claimed to prove ethical approval by Nigerian authorities for the trial appeared to be falsified and back-dated.
In March 2009, Pfizer and Kano State officials, along with representatives of the children’s families, met to confirm an out-of-court settlement for US $75 million, with a confidentiality agreement. The conclusion of the trial by a settlement led to compensation for some of the families affected, but Pfizer never admitted to wrongdoing, and maintains to this day that the trial was proper and life-saving.
Who Makes a Good Test Subject?
“Kano was probably chosen because of the huge population, high prevalence of meningitis,” Dr. Mohammed of the College of Medicine at the University of Jos explained, “and the people’s attitude of always accepting bad outcomes as the will of God, which makes it easier to cut corners.”
But beyond the religious and cultural dynamics, he also pointed to the fact that “One hardly ever hears of malpractice suits in this part of the country [Northern Nigeria]”.
In a study entitled “Ethics of Clinical Trials in Nigeria,” Dr. Patrick I. Okonta of Delta State University, Abraka, Delta, Nigeria, found that developing countries offer considerably attractive conditions for companies hoping to minimize the price of pharmaceutical trials.
“Pharmaceutical companies are business companies and like all business companies, their main objective is to maximise profit while reducing cost of production. …Developing countries hold a large reservoir of diseases and the required number of patients can be recruited within a short duration. Also, compensation for lost time and wages paid to research participants in developing countries is small compared to what is payable to research participants in developed countries.” – Dr. Patrick I. Okonta
The International Ethics of Using Humans as Test Subjects
At the College of Medicine, University of Jos, Dr. Sabo Ahmed Mohammed told Unbias the News that the 1996 trial was in contravention of both local and international ethics. “There were unethical practices against the Nuremberg Code, the Helsinki Declaration and our [Nigeria’s] national laws on bioethics”.
The Nuremberg Code is a set of principles concerning the use of human test subjects in experiments, developed after the horrific experiments conducted by doctors of the German Third Reich on prisoner subjects.
The Helsinki Declaration of 1964 similarly requires researchers to put the health of human research subjects first. Both international standards, however, have not seemed to prevent pharmaceutical companies from engaging in questionable practices in Nigeria and Africa more widely.
The Nuremberg Code is part of the international systems that emerged after World War II, with its focus on respect for human rights, individual autonomy, and informed consent. In the aftermath of world war 11 in 1945, International Military Tribunal held a series of tribunals against major war criminals and Nazi sympathizers. The verdict then led to the creation of the Nuremberg Code.
International legal standards are only effective insofar as they can be enforced, and this often depends on local or domestic authorities.
Professor Remigius N. Nwabueze of the University of Southhampton, UK, finds that despite Nigeria’s involvement in biomedical research since colonial times, Nigeria does not have any formal framework for regulating research involving human participants.
In his research, he identifies the National Institute of Medical Research in Yaba, Lagos (NIMR) as the organization authorized to conduct medical research related to health problems in Nigeria. Though the NIMR is a major Nigerian institute concerned with human medicine and research in Nigeria, it has not promulgated any formal guidelines for the conduct of research involving human subjects, he argues.
Although the Nuremberg Code and the Declaration of Helsinki apply in Nigeria, they are “without the benefit of an implementing and elaborating domestic regulatory instrument”. He concludes,
“Under-regulation increases the potential risk of exploitation in Nigeria by international corporations seeking clinical trials in countries with zero or minimal regulation.”
Doses of Vaccines Destroyed
Many years later, the memory of the Pfizer Trovan drug trial remains and for most of the affected families, the COVID-19 vaccine is a reminder of the lingering doubts over whether big pharmaceutical companies cast ethical concerns aside in places like Kano. Concerns about vaccine safety may be associated with vaccine hesitancy.
“I won’t advise, I won’t allow and I won’t tolerate seeing my son, myself or any of my relatives to receive the COVID-19 vaccine” – Maryam maintains.
She vows to discourage anyone she knows from taking the vaccine and if said persons aren’t aware of the 1996 meningitis outbreak and Pfizer’s Trovan, “I will educate them on that. My son is now living in agony despite the so-called compensation”.
“He is neither in school nor into business. He is living a miserable life,” she added.
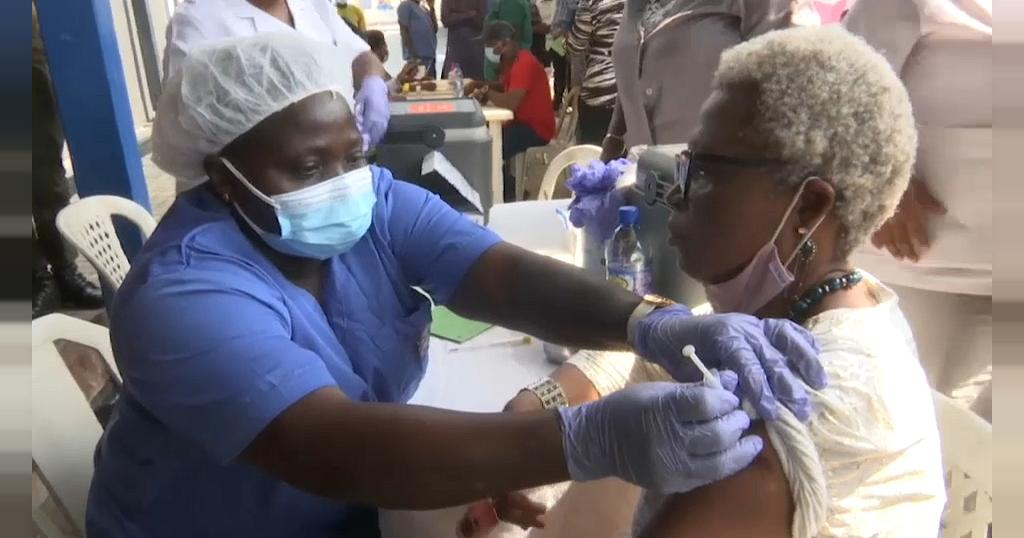
Maryam is not alone in her doubts. Some Africans are hesitating to get COVID-19 vaccines amid concerns about their safety. From Congo to Malawi and South Sudan, doses of the expired vaccines have been destroyed, a development that raises concerns for vaccine equity and the effectiveness of a global vaccination effort that requires mass participation to be effective.
Dr. Samaila Suleiman, a lecturer of History at the Bayero University Kano, during an interview with Unbias the News, argued that the skepticism over COVID-19 vaccine can be traced to historical cynicism against the motives of western industrialized powers in Africa.
“It is important to also note that the COVID-19 vaccine skepticism is not peculiar to the uninformed members of the community”. Suleiman continued, “there are highly placed members of the elite and political class who have refused COVID-19 vaccine, citing a western conspiracy to decimate the African population”. – Dr. Samaila Suleiman
Theories like these continue to flourish, from religious leaders in the country who continue to advocate and lobby worshippers against taking the vaccines as it leads to a 5G frequency radiation, to those who argue that the virus is a hoax by big pharmaceuticals to profiteer off vaccine sales.
Can Transparency Fight Hesitancy?
Some Western nations are not only sufficient in distribution, they are also rolling out incentives, from $1 million cash prizes to a free dinner and education scholarships as part of their efforts to boost vaccination rates.
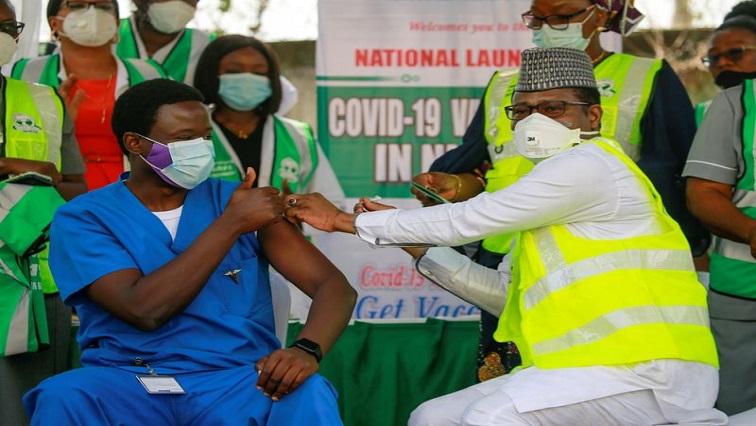
In situations where vaccine safety is one of the underlying causes of vaccine hesitancy what are the chances for these communities? “We recognize that vaccine hesitancy is a global phenomenon,” Dr. Faisal Shuaib, ED/CEO of the Nigerian National Primary Healthcare Development Agency opined, “as public health experts, we must do more than offer the vaccine”.
“We have to also put in the hard work of providing the correct information about the safety, effectiveness of vaccines and clear the doubts and misconceptions that exist,” he added.
These doubts may be difficult to disprove when pharmaceutical companies remain unrepentant for previous mistreatment of patients in Africa and elsewhere, settling disputes with out-of-court payments cloaked in secrecy. In the case of the Kano State, part of the damages requested from Pfizer included funds to educate the public and dispel myths about the safety of Western medicine.
But as large pharmaceutical companies may continue to lack transparency and accountability, countries can also take measures to hold them to account. A sign of hope in this regard comes from a recent case in Uganda. The high court only recently, in the case Mukoda v International AIDS Vaccine Initiative, set out guidelines for obtaining informed consent from the subjects of human clinical drug trials.
Mukoda v. International Aids Vaccine Initiative
“In any research on human beings, each potential subject must be adequately informed of the aims, methods, sources of funding, any possible conflicts of interest, institutional affiliations of the researcher, the anticipated benefits and potential risks of the study and the discomfort it may entail.”
For 29-year-old Bello and Maryam, the 1996 meningitis outbreak and its after effect remains a bitter pill to swallow and bolsters intense skepticism about the Covid-19 vaccine and the pharmaceutical industry in general.
“I am skeptical about taking any vaccine now. I won’t take COVID-19 vaccine from Pfizer or a different pharmaceutical company,” Bello reiterates.
As health advocates struggle to fight disinformation and conspiracy theories about the COVID-19 vaccines, it is important to remember that in some countries distrust stems not only from ignorance, but also from experience.
Subscribe
Be the first to receive special investigative reports and features in your inbox.