The National Agency for Food and Drug Administration and Control (NAFDAC) has warned healthcare providers and the public against the use of dexamethasone tablets, a pain relief medicine, after it was found out that some substandard drugs had been purchased in Anambra State.
In a statement released on Sunday by the agency, Nigerians were advised to be on the lookout for the substandard dexamethasone tablets as it attempts to mop up the ones that are already in circulation in the country.
“This recall is due to unsatisfactory results of analysis which showed that the samples were found to contain substandard amounts of dexamethasone (i.e. less than the amount stated on the labels),” the agency stated on its website.
“Some of the samples were also found to contain methylparaben and/or propylparaben in small amounts. Methylparaben and/or propylparaben is a preservative often used to give a product a longer shelf life.
“The presence of these preservatives in tablets are unusual and unnecessary and the use of these substances should be avoided, particularly in case of pediatric formulations.”
READ ALSO: DO NOT USE: 4 Cough Syrups Made in India Have Killed 66 Children in Africa
The agency also disclosed that the use of the substandard drugs could cause harm to patients.
“The administration of the subtherapeutic doses of dexamethasone in the products or any substandard and falsified medical products may cause harm to patients and lead to treatment failure,” the agency wrote.
“They can also lead to loss of confidence in medicines, healthcare providers and health systems.”
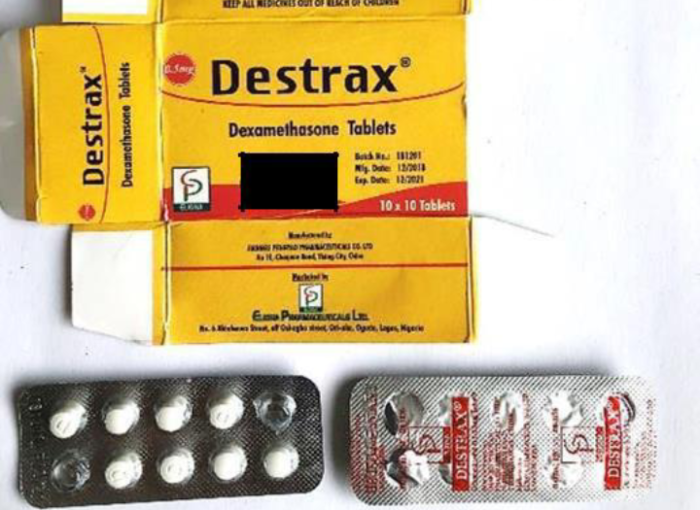
NAFDAC also listed other dexamethasone tablets that the public should also avoid buying and they include Destrax, Dexacotin, Dexacure, Dexalab and Xasten.

READ ALSO: Japata Bitters, a Nigerian Alcoholic Drink, Kills Mice at Testing
The agency directed all concerned Marketing Authorisation Holders (MAH) of the affected brands to initiate a nationwide recall of their products with immediate effect and submit updates of the recall to the agency for effective monitoring.
“The agency has heightened surveillance in the 36 States and the Federal Capital Territory, Abuja, to stop the distribution and sale of the above listed dexamethasone products,” the statement read.
“All distributors, wholesalers, retailers and consumers in possession of the recalled products are implored to discontinue sale or use and submit stock to the nearest NAFDAC office.”
Dexamethasone provides relief for inflamed areas of the body. It is used to treat a number of different conditions, such as inflammation (swelling), severe allergies, adrenal problems, arthritis, asthma, blood or bone marrow problems, kidney problems, skin conditions, and flare-ups of multiple sclerosis.
Subscribe
Be the first to receive special investigative reports and features in your inbox.